Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Assessment of therapeutic response and monitoring for relapse are important components of clinical care in this entity. Although circulating tumor DNA (ctDNA) has shown to be a potential biomarker, it is affected by gene dysregulation and limited by cost, as it requires high amplification, especially in early stage DLBCL since only ~100 ng free DNA fragments are detected in 1 mL blood. Extracellular vesicles (EVs), on the other hand, are abundant in the blood (1011 - 1012 in 1 mL blood), making quantitative analysis easier and less expensive. This OSU based technology has been extensively studied in lung and pancreatic cancer as well as chronic lymphocytic leukemia and multiple myeloma with promising results. Hence, we sought to evaluate whether Immune Lipoplex Nanoparticle (ILN) biochip-based testing (the first of its kind to monitor patients with lymphomas) can serve as a useful means to follow select biomarkers for monitoring therapeutic response.
Methods
This is a single-center prospective study of de-novo DLBCL patients wherein the patient samples were longitudinally collected before, during, and after chemotherapy upto 2 years every 3 months. The patients were classified as responders if they achieved complete remission (CR) to chemotherapy following cycle 6 (C6), and non-responders/primary progressors if they had progression.
In ILN biochip assay, tethered antibodies on the chip surface can capture and sort an EV subpopulation rich with specific membrane proteins after washing. The fluorescence labeled antibodies or cationic lipoplex nanoparticles (CLNs) containing molecular beacons (MBs) are then added to fuse with captured EVs for the detection of membrane protein or mRNA targets respectively.
Well known B-cell markers (CD10, CD19, CD20, MUM1, BCL2, BCL6, and cMYC) and a relatively new lymphoma biomarker, CD47 was measured in plasma samples from DLBCL patients before and after chemotherapy with ILN biochips. EV subpopulations rich in CD20 and/or CD19 (DLBCL markers) or CD63/CD9/CD81 (exosome markers) were captured by antibodies tethered on the biochip surface. Subsequently, ILN biomarker measurements were obtained for CD10, CD19 and CD20 protein expressions by fluorescence-labeled antibodies on anti-CD19/CD20 captured B-cell dominated EV subpopulation; MUM1, BCL2, BCL6, cMYC, and PRMT5 mRNA expressions by MBs encapsulated in CLNs in anti-CD63/CD9/CD81 captured exosome-dominated EV subpopulation; and CD47 protein expression by fluorescence-labeled antibodies on the anti-CD47 captured/ CD47-rich EV subpopulation.
Results
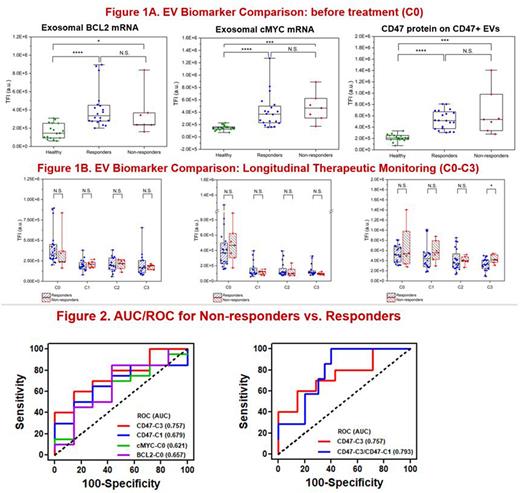
For plasma samples taken before treatment (C0), the exosomal cMYC, BCL2, and BCL6 mRNA expressions could discriminate between healthy donors (HDs, n=17) and patients (n=27), while other B-cell markers did not perform well (Figure 1A). Since BCL2 and BCL6 detection demonstrated similar trends with BCL2 performing slightly better, exosomal cMYC and BCL2 mRNA expressions were selected as the biomarker classifiers. CD47 protein expression by CD47-rich EVs also discriminated between HDs and patients well in pre-treatment (C0) samples (Figure 1A). During chemotherapy, the EV CD47 expression demonstrated a greater decrease in responder samples (n=20) than in non-responder samples (n=7) obtained after C3 showing a statistically significant difference (Figure 1B). Longitudinal CD47 expression (i.e., C1 and C3) on CD47+ EVs could serve as a potential biomarker to discriminate between responders and non-responders of DLBCL patients undergoing chemotherapy with AUC~0.80 (Figure 2). The results of the longitudinal tri-biomarkers, i.e., CD47(C3)/cMYC(C0)/BCL2(C0) expressions will be presented at the meeting.
Conclusions
We discovered a new longitudinal tri-biomarker classifier based on CD47/cMYC/BCL2 expression after early chemotherapy treatment (C0 and C3) for monitoring chemotherapy response in DCBCL. However, the sample size, particularly in the non-responder cohort (n=7) is small to make any statistically meaningful conclusions. Additional patient samples are being collected to validate these findings. Lastly, to improve the biomarker classifier performance for chemotherapy monitoring (and maybe relapse surveillance), we are conducting an EV mRNA profiling study to identify novel biomarker candidates.
Disclosures
Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Bond:SeaGen: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria; Nurix: Research Funding; Novartis: Research Funding. Sawalha:Epizyme: Consultancy; BeiGene: Research Funding; Celgene/BMS: Research Funding; TG Therapeutics: Research Funding. Reneau:Incyte: Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; ADC Therapeutics: Research Funding; Merck: Research Funding; Corvus Pharmaceuticals, Inc.: Research Funding; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees. Voorhees:Incyte: Research Funding; AstraZeneca: Research Funding. Maddocks:Lilly: Consultancy; Kite: Consultancy; Genmab: Consultancy; Beigene: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Morphosys: Consultancy; ADC Therapeutics: Consultancy; Gilead: Consultancy; BMS: Consultancy, Research Funding; Abbvie: Consultancy; Pfizer: Research Funding; Janssen: Consultancy; Pharmacyclics: Consultancy, Research Funding; Acerta: Consultancy. Christian:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/Bristol-Myers Squibb: Research Funding; Acerta: Research Funding; Triphase: Research Funding; Millennium: Research Funding; Seattle Genetics: Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Baiocchi:Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CODIAK Biosciences: Research Funding; eLife (Journal): Other: Editorial board; Viracta Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal